
FAQ: Questions & Answers
FAQ related to the Field Safety Notice published in November 2023
FAQ: Questions & Answers
The below FAQ has been updated on 26.01.2024
We kindly ask you not to share this FAQ with any of your external stakeholders such as users of Actim® PROM, Actim® PROM 1ngeni, and/or Actim® Partus.
This FAQ is only intended to be used as a support tool for you as a distributor so that you can have answers to the different questions your customers might have.
Please note that this FAQ is related to Actim® PROM, Actim® PROM 1ngeni, and Actim® Partus only. It does not apply to Check PROM.
FAQ related to the Field Safety Notice on Actim® PROM, Actim® PROM 1ngeni and Actim® Partus tests regarding lubricants published in November 2023.
Q1: Have any changes been made to Actim PROM, Actim PROM 1ngeni, and/or Actim Partus, as this interference has not been claimed before?
No, no changes have been made to the Actim PROM, Actim PROM 1ngeni or Actim Partus rapid tests. The reason why the interference of lubricants with the test results has not been claimed before is that the initial interference studies have been limited. The risk of interference has been identified in the study that has recently been conducted, where several lubricants were tested.
Q2: May this interference be causing false positive or false negative results?
Different lubricants may have varying interfering effects on the test results. Therefore, the outcome of this interference may be false positive or false negative results.
Q3: What kind of lubricants have been found to cause interference with the test results in your studies?
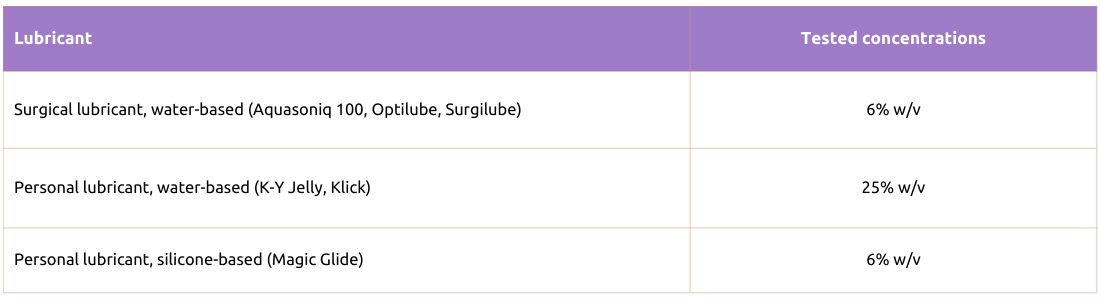
Actim Partus and Actim PROM rapid tests have been tested with water-soluble surgical lubricants (Aquasoniq 100, Optilube, Surgilube, HR Lubricating Jelly) and personal lubricants (K-Y Jelly, Klick).
Q4: Is there a lubricant that would be safe to use, or a known concentration for which the lubricants do not interfere with the test results?
Care must be taken not to touch anything with the swab before taking the specimen. Do not contaminate the swab or cervicovaginal specimen with lubricants or creams, as they may physically interfere with absorption of the specimen onto the swab and/or affect the test performance.
The following lubricants were tested with Actim PROM test and Actim Partus test and were found not to affect their performance, when tested at the concentrations shown below:
Table 1. Actim PROM
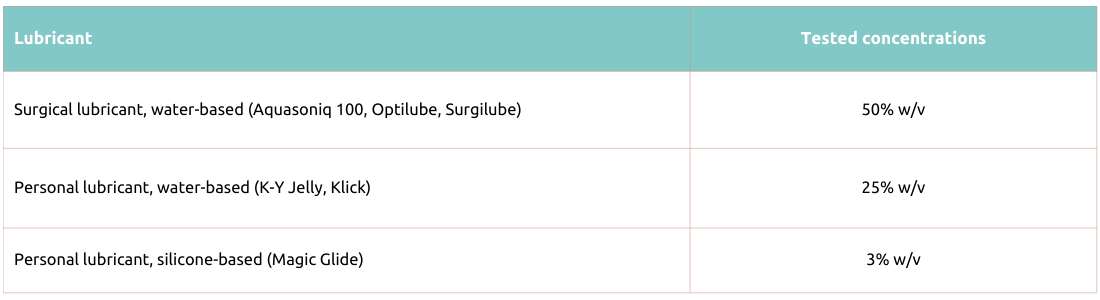
Table 2. Actim PROM 1ngeni
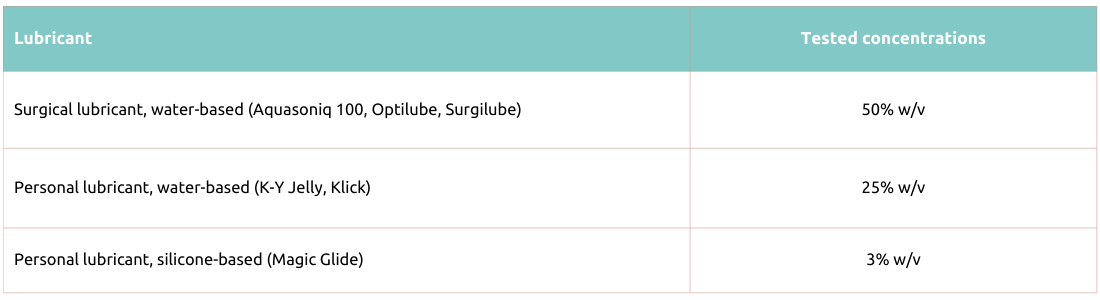
Table 3. Actim Partus
Q5: When are you planning on updating the instructions for use (IFUs) related to those rapid tests?
The instructions for use will be updated as soon as possible. Meanwhile, a separate leaflet will be included into the test kits to notify customers to refrain from using any lubricants.
Q6: If there is a concern that specimens that have recently been collected may have included some lubricant, should the tests be repeated?
Rapid tests are used as assisting tools to support timely clinical decisions. Therefore, repeating the test later may no longer be relevant to the initial clinical situation. The need for further testing should be considered by the responsible healthcare professionals.
Q7: What type of lubricant do you mean by “surgical lubricant”?
A surgical lubricant is a substance used by healthcare professionals to provide lubrication and hence decrease the discomfort of the patient during certain medical examinations, including vaginal examinations.
It is a common practice for healthcare professionals to use a surgical lubricant during an examination that involves the use of a speculum. Examples of surgical lubricants include Optilube and Aquasonic 100.
Q8: What type of lubricant do you mean by “personal lubricant”?
A personal lubricant is a consumer product that can be used e.g. during sexual intercourse. Examples of personal lubricants include K-Y Jelly and Klick.
Q9: Does this change — refrain from using surgical and personal lubricants — mean that the sales and marketing materials related to Actim PROM, Actim PROM 1ngeni, and/or Actim Partus need to be updated?
Yes, the sales and marketing materials related to Actim PROM, Actim PROM 1ngeni, and/or Actim Partus and stating that lubricants do not interfere with the test results must be deleted.
Q10: What alternatives to lubricants can be used for speculum lubrication?
It is not necessary to use a lubricant for speculum insertion. Water or saline are commonly used to lubricate the speculum and they can be recommended as alternatives.
In our testing, baby oil (Natusan) and skin oil (Ceridal) were tested with a concentration level in the specimen of up to 50% and no interference was found. Thus, modest lubrication of the external surface of the speculum with Natusan or Ceridal oil is not expected to affect the test results of Actim Partus, Actim PROM or Actim PROM 1ngeni. However, care must be taken not to contaminate the swab with any oil when proceeding with the specimen collection.
Furthermore, please note that when it comes to Actim PROM and Actim PROM 1ngeni, the specimen can be collected with or without the use of a speculum. The use of a speculum is only required to collect a specimen when Actim Partus is the rapid test performed by the healthcare professional.
Important note: The specimen collected for the Actim PROM or the Actim Partus test must be collected before performing a digital examination and/or a transvaginal ultrasound, as mentioned in the current version of the IFU.
Q11: How much time needs to pass after the lubricant has been used until the specimen can be extracted so that there would not be any risks of interference with the test result?
The vagina is a self-cleaning organ where vaginal discharges actively flush the vaginal canal. It is difficult to state the exact time when lubricants have been flushed out completely. However, it is unlikely that any lubricant would be present in the vagina after 24 hours.
Q12: How far back in history should we go when informing customers?
This information is relevant to those customers who may have valid kits and are actively using the tests.